
First, identify the reactants and determine if they are a metal or a halogen. The steps to determining a product are simple.
MEMORIZE ACTIVITY SERIES OF METALS SERIES
How do you use activity series to predict products? A precipitation reaction is a double-replacement reaction in which one product is a solid precipitate. A double-replacement reaction exchanges the cations (or the anions) of two ionic compounds. The periodic table or an activity series can help predict whether single-replacement reactions occur. I LIKE HIS CAR’S SILVERY GATE.ĭo you use the activity series for double replacement? Short Trick to Remember Reactivity Series “Please send charlie’s monkeys and zebras in lead & hydrogen cages in mountains securely guarded by Plato.” In the above-given sentence, first alphabet of every word denotes the elements of the reactivity series in order of their reactivity from highest to lowest. What is the trick to learn the reactivity series? I’d learn it just to stay on the safe side, when I did GCSEs we had to learn the reactivity series so I don’t know if it’s changed for you. It says you need to “explain” things by using the reactivity series but it doesn’t say you have to “recall” it. It can also be used to obtain information on the reactivity of metals towards water and acids.ĭo you need to memorize the activity series? The data provided by the reactivity series can be used to predict whether a metal can displace another in a single displacement reaction. When should you use the activity series how do you use it? The activity series including these elements would be Mg > Zn > H. so zinc is also more active than hydrogen. What is an example of activity series?Īn activity series is a list of substances ranked in order of relative reactivity. It can be used to predict the products in similar reactions involving a different metal.
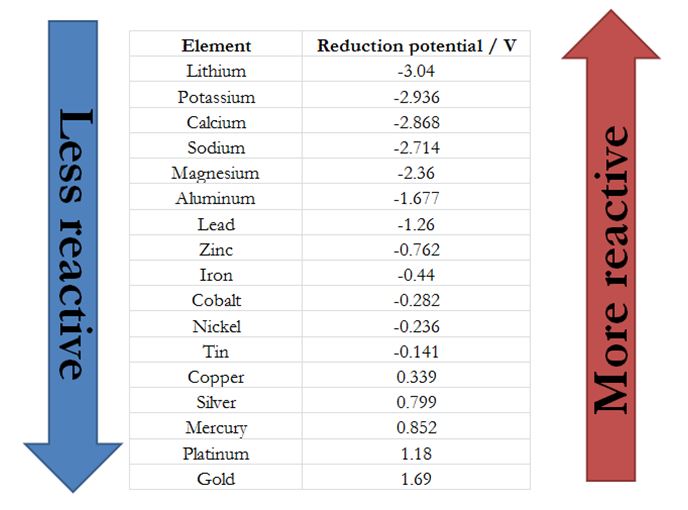
The activity series of metals is an empirical tool used to predict products in displacement reactions and reactivity of metals with water and acids in replacement reactions and ore extraction.

How is the activity series used in predicting products of chemical reactions?

I also saw "Nick the Camel." in the replies. I prefer LEO goes GER to OIL RIG because it is clear electrons are what is lost/gained. SOAC a GERC (soak a jerk) makes me laugh Strongest Oxidizing Agent at the Cathode Gains Electrons and is Reduced at the Cathode.

OILRIG Oxidation Is Loss, Reduction Is again- Carolyne March 14, 2018 Have No Fear Of Ice Cold Beer! (diatomic)- Chuck Boland MaElectrolysis made easyĪ colleague of mine uses PPOO (“stuttering poo”) for electrode potentials - Positive Potentials Oxidise Others!- Peter Hoare March 14, 2018Ĭathode- A-Level Chemistry 🎓 March 14, 2018 I Have No BRight Or CLever Friends- Lindsay Turk March 14, 2018 I do 'Clevland Brown Has No Friends In Ottawa'.- Sherry Lynn McGregor March 14, 2018 SEA GIL strong electrostatic attraction, giant ionic lattice.- Elizabeth Keay MaDiatomic molecules? You say ’hobrfincl’ and I say ’brinclhof’ My colleague's SEABOCI "strong, electrostatic attraction between oppositely charged ions"- Kristy Turner March 13, 2018 favourite mnemonics to help kids recall things? Here are a few crackers for remembering some of the subject’s tricky details. A flurry of nifty aide-memoires were tweeted back. This week, Kristy Turner asked for your favourite chemistry mnemonics.


 0 kommentar(er)
0 kommentar(er)
